What is PMM2-CDG?
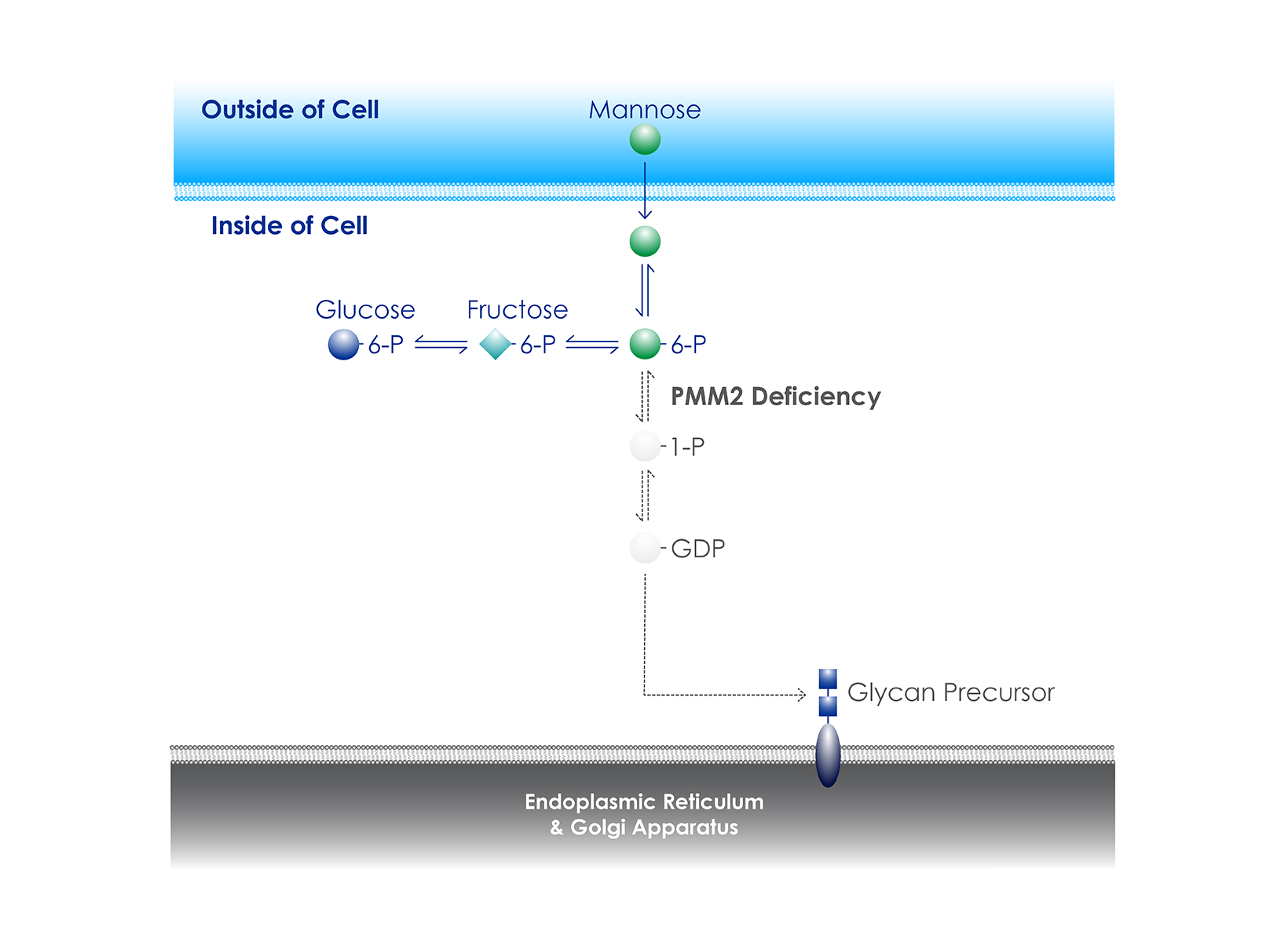
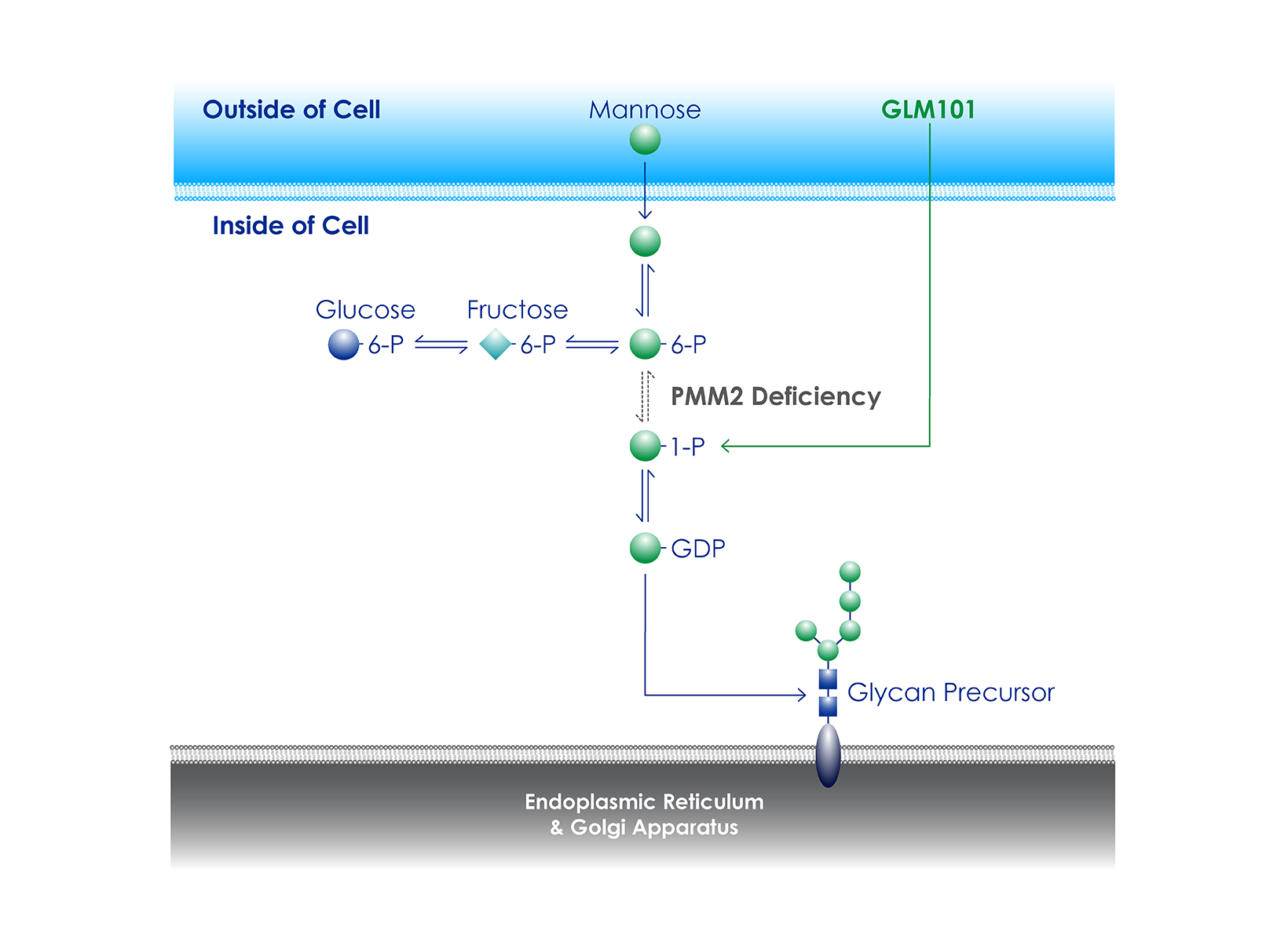
PMM2-CDG (also known as CDG-1A or CDG Type 1a) is a rare genetic disease of glycosylation. While currently approved therapies only treat symptoms and are not disease-modifying, Glycomine hopes to change that with the clinical development of GLM101, a mannose-1-phosphate replacement therapy.